Battery Science: What’s next?
In the midst of the continuously growing gigawatts of installed battery capacity, lithium-ion batteries (LIB) have risen as the predominant choice. When contrasted with alternative battery compositions, lithium-ion batteries undeniably present the most appealing blend of energy density and cost efficiency.
Yet, the quest for ever more energy density and the limitations imposed by mineral resources – like cobalt or lithium itself – have forced battery experts to continuously work on innovations and alternatives. Among the extensive list of news we have been hearing over the last few years, four tendencies seem to be settling as dominant technologies for the next decade:
- Lithium-ion (Li-ion) positive electrode optimization
- Li-ion new negative electrode composition
- Lithium metal based solid-state batteries (SSB)
- Sodium-ion (Na-ion) batteries
In this edition of Battery Knowledge, we will explore the benefits of such innovations as well as their limitations and challenges.
LI-ION, THE QUEST FOR DENSITY
While grid-scale battery energy storage is expanding its market presence and influence, the primary impetus behind battery innovation presently stems from the demands of electric vehicles (EVs), particularly in terms of power and energy output. Consequently, all advancements in battery technology are oriented toward achieving higher energy density and increased power capabilities to meet these requirements.
Why? High energy implies the capacity to hold a significant amount of energy with minimal weight and volume, which is particularly advantageous when developing an EV or even in some cases BESS.
On the other hand, part of the incentive arises from the fluctuating prices of battery components, which could incentivize companies to explore different chemical compositions. Positive electrodes usually account for a significant portion of a battery’s expenses, and the prevailing positive electrode types are NMC (Nickel Manganese Cobalt) and LFP (Lithium Iron Phosphate). The costs associated with these three elements, along with lithium, can be substantial.
In this context, battery manufacturers continuously tend to improve the component of existing technologies and new generations of NMC and LFP batteries. For more information about battery chemistries, read NMC vs LFP article.
I- Improving the existing technologies: positive electrode
a. C/NMC, the gifted child
At first glance, the exceptional energy density, abundant power availability, and effective low-temperature functionality of NMC cells positioned them as an ideal choice for EVs.
Yet, there is an issue with NMC and its use of Cobalt: Cobalt is considered a rare metal, it is not abundant, its cost is prohibitive, and its localized extraction is a risk for countries sovereignty and safety of production.
Ni-rich NMC, more nickel in the mix
Until a few years ago, NMC batteries comprised approximately equal parts of nickel, manganese, and cobalt, denoted as NMC333. As time progressed, significant strides were made in reducing cobalt content, leading to formulations such as NMC532 (consisting of 50% nickel, 30% manganese, and 20% cobalt). In the present day, the evolution continues with the development of NMC811 and its further reduced cobalt content.
As the amount of nickel present in the electrode increases, its energy density also rises. In the near future, we expect to see even more Ni-rich NMC with NMC 955 (90% 5% 5%) and even NMC 9525 (95%, 2.5%, 2.5%).
However, this fine-tuning of NMC doesn’t address all challenges. While these improvements hold great potential in terms of energy density and cost reduction, the presence of manganese and cobalt plays a crucial role in stabilizing the electrode. Their removal diminishes cell safety: the onset temperature for thermal runaway is lower in these subsequent iterations, adversely impacting their lifespan.
b. C/LFP, forever second?
LFP stands as a strong contender against NMC, addressing certain vulnerabilities: it boasts enhanced safety, facilitates quicker charging, and employs more cost-effective materials. Nevertheless, its drawback lies in its lower energy density: ongoing efforts are dedicated to enhancing its cell voltage by incorporating new metals into the crystal structure.
Manganese introduction, the new LMFP
One approach involves introducing manganese, resulting in an electrode referred to as LMFP (e.g., Gotion with a capacity of 180-220Wh/kg at 4.1V). In that sense, CATL recently unveiled a novel LFP variant named M3P, featuring a blend of at least three undisclosed metals among which manganese. This innovation is anticipated to yield improved stability and faster charging capabilities, to be continued…
But again, the industry must be cautious as adding other metals to the electrode may destabilize it. Manganese is known to be prone to dissolution and deposition on the negative electrode, which can lead to heavy battery degradation, eventually leading to premature end of life.
With these risks, a refined monitoring of battery degradation is a must to ensure battery asset performance and prevent early arrival to the system’s knee-point.
II- Improving the existing technologies: negative electrode
Graphite had been serving since the 1980s as a commendable material of the negative electrode due to its capacity to accommodate a single lithium ion per every six carbon atoms, with a density of 360mAh/g. Today, silicon appears to be more and more an interesting alternative with its 1300mAh/g.
The switch from graphite to silicon is however not easy: silicon particles swell during the charge of the battery and their size increases vastly (+100%). It creates deformations that lead to huge constraints in the Solid Electrolyte Interface (SEI) and the particles, eventually accelerating the loss of lithium inventory (LLI) and thus the aging of the cell. To learn more about SEI and LLI, refer to the previous article on battery degradation.
But silicon high potential makes manufacturers looking for ways to prevent these deformations: the two main solutions are either to coat graphite particles – less prone to deformations – with silicon nano particles (see fig.1 ) or to encapsulate silicon inside bigger porous carbon particles on which the SEI forms to protect it (see fig.2).

Figure 1. Silicon coating of a Graphite particle

Figure 2. Silicon encapsulation in porous Graphite sphere (Source: Crumpled Graphene-Encapsulated Si Nanoparticles for Lithium Ion Battery Anodes, Jiayan Luo et al)
Will we soon end up using with Si/NMC & Si/LFP?
SOLID STATE, SAFETY & DENSITY
Well well, all these improvement on LIB seem to not be enough, batteries are gaining energy density – not huge progress still – but suffering from lack of stability and safety. The question is, how can we get the maximum energy density without compromising its stability?
In the context of LIB, the highest-density negative electrode available to us is metallic lithium itself, boasting a density of 3800mAh/g compared to 1300 mAh/g for silicon. While lithium metal electrodes have a history of use in primary cells, they present distinct challenges when used for cycling. The creation of a stable SEI layer becomes indeed problematic due to their significant volume changes during cycling.
Additionally, controlling their deposition behavior during charging is challenging, often resulting in the formation of dendrites: accumulation of lithium into formations resembling spikes, somewhat akin to stalactites. Over time, these accumulations can puncture the battery separator, leading to a short circuit between the anode and cathode.
To effectively utilize such electrodes, a significant obstacle must be addressed: the adoption of solid electrolytes.
Introduction of Solid-State Batteries (SSB)
Currently, research has identified three primary categories of potential candidates for solid electrolytes:
- Polymers: these represent the sole type of solid electrolyte that is currently industrially viable. However, they exhibit limited stability at high potentials and are consequently used currently in conjunction with LFP. Polymers also require elevated temperatures (around 80°C) for efficient ion conduction, which can reduce battery autonomy and add complexity to the pack. Additionally, they operate under high pressure conditions.
- Sulfides: similar to polymers, sulfide-based electrolytes face challenges with stability at high potentials, which restricts their use with the most energy-dense positive electrodes. They also pose difficulties in large-scale production and can encounter interface issues when paired with lithium metal electrodes.
- Oxides: oxide-based electrolytes have the advantage of overcoming the high potential limitations seen in the other two categories, making them strong candidates for future battery generations. However, their current performance levels are quite limited, and they present processing challenges that need to be addressed for widespread adoption.
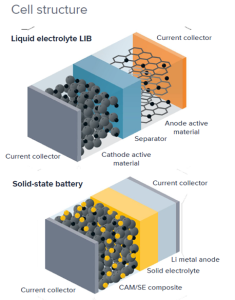 *
*
Figure 3. SSB cell structure in comparison with LIB (Source: Solid-State Roadmap Fraunhofer)
It’s worth noting that it is possible to combine different types of solid electrolytes as long as they can interface effectively. In such cases, they are referred to as catholyte (positive electrode electrolyte) and anolyte (negative electrode electrolyte).
Solid-state batteries hold significant promise and are poised to soon reach an energy density of 400 Wh/kg, with a target of 500 to 550 Wh/kg by 2030.
What about safety?
SSB should offer improved safety, as they lack flammable electrolytes, reducing the risk of fires despite metallic lithium high reactivity to water/humidity. Nevertheless, substantial work is still needed to optimize their performance under “normal conditions,” defined as 25°C and low pressure.
Anyway, after a first successful experience monitoring of solid-state Lithium Metal Polymer battery, PowerUp innovation team is looking forward to having more and more SSB batteries in operation.
NA-ION BATTERY, QUEEN OF SUSTAINABILITY
At this point, you understand that whether on the electrodes or on electrolytes, every effort put in innovation is made to enhance batteries energy density and offer more power to battery assets. But, is it that important to reach high levels of energy density? Is it necessary for all battery applications?
Lithium is the best element to store electricity, it is light and has a natural tendency to shed electrons when it participates in chemical processes. But others can be used with sufficient performance.
In the periodic table of elements, elements in the same column have similar properties and guess what? The next elements in lihtium column is sodium (Na). Sodium is a bigger atom (11 nucleons vs 3) which presents challenges for the insertion in electrodes and the famous rocking chair principles of LIB.
Figure 4. Periodic table of the elements
The size of sodium imposes a change in the usual graphite negative electrode: bigger pores are needed and we have to use hard carbon. In terms of positive electrodes, two types can be used: either ferrous cyanide, with a Prussian blue structure (cells around 100Wh/kg) or lamellar oxides (cells around 150 Wh/kg – which is close to current LFP densities).
Sodium like Salt?
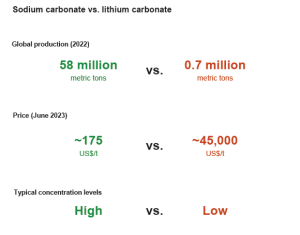
Sodium-ion batteries present many advantages compared to LIB. First regarding supply, there’s salt everywhere!
Sodium is indeed abundantly available worldwide, in contrast to lithium, which is concentrated in a few countries. This widespread availability of sodium presents a substantial geopolitical advantage for every nation. On the other hand, the materials employed in sodium-ion batteries are notably cost-effective, with a substantial price difference between sodium carbonate and lithium carbonates.
Yet, manufacturers will have to reach an economy of scale to ensure the competitivity of Na-ion against settled Li-ion technologies: same dilemma as between LFP and NMC. Even though materials in LFP were expected to be less expensive, the NMC prices per kWh remains lower due to economies of scale and high-density cells.
Figure 5. Sodium abundance in comparison with Litihum (Source: S&P Global Commodity Insights)
When it comes to operation,
- Extreme temperatures don’t impact lifetime
Na-ion operates effectively in a broader temperature range, spanning from -40°C to 80°C, as opposed to the -10°C to 60°C range for lithium-ion batteries. Under these conditions, sodium-ion batteries exhibit a similar lifespan to lithium-ion batteries.
- Safety is barely a question
Na-ion is considered safer, with a significantly reduced likelihood of thermal runaway, and they can be discharged down to 0V, enhancing their safety during transportation.
Battery chemistries: What to expect in the future?
More energy density with elevated Li-ion batteries,
- Li-ion positive electrodes optimization: the positive electrodes optimization will help gaining more and more energy density and reduce cobalt dependency. Yet, Nickel enriching for NMC and Manganese introduction for LFP are to monitor with caution as it compromises stability and raise safety concerns.
- Silicon as new negative electrode: silicon is emerging as an increasingly compelling alternative due to its 1300mAh/g capacity. Nonetheless, transitioning from graphite to silicon poses challenges, as silicon particles undergo significant swelling during the battery’s charging process, that can lead to accelerated degradation of the battery.
Even more energy density and safety with SSB,
- Lithium metal based solid-state batteries (SSB): Solid-state batteries exhibit considerable potential and are on the verge of achieving an energy density of 400 Wh/kg, while maintaining a high level of safety.
Potential high degree of sustainability, both economically and environmentally, with Na-ion.
- Sodium-ion (Na-ion) batteries:if economies of scale prove effective, Na-ion technology could present a compelling alternative to Li-ion batteries. While Na-ion cells may have slightly lower energy density, they offer high stability and temperature adaptability.